The First North company ViroGates may be just two-three months away from an outright sales boom. Swedish drug company Sobi, whose product Anakinra can cure potentially fatal Covid-19 patients, last week submitted an application to the European Medicines Agency (EMA) that could have a huge impact on Virogates.
ViroGates’ commercial success with its corona marker is entirely dependent on Sobi, which has just submitted a marketing application to the EMA. European approval of Sobi’s product, which can cure very sick Covid-19 patients, is expected as early as October, after which commercial roll-out is expected immediately afterwards.
The link between Sobi and Virogates is as follows: Sobi’s product Anarkina has been on the market for almost 20 years, and is only used in very sick people. Due to serious side effects, in the case of Covid-19, the product will only be used in patients who are either very ill or expected to become very ill.
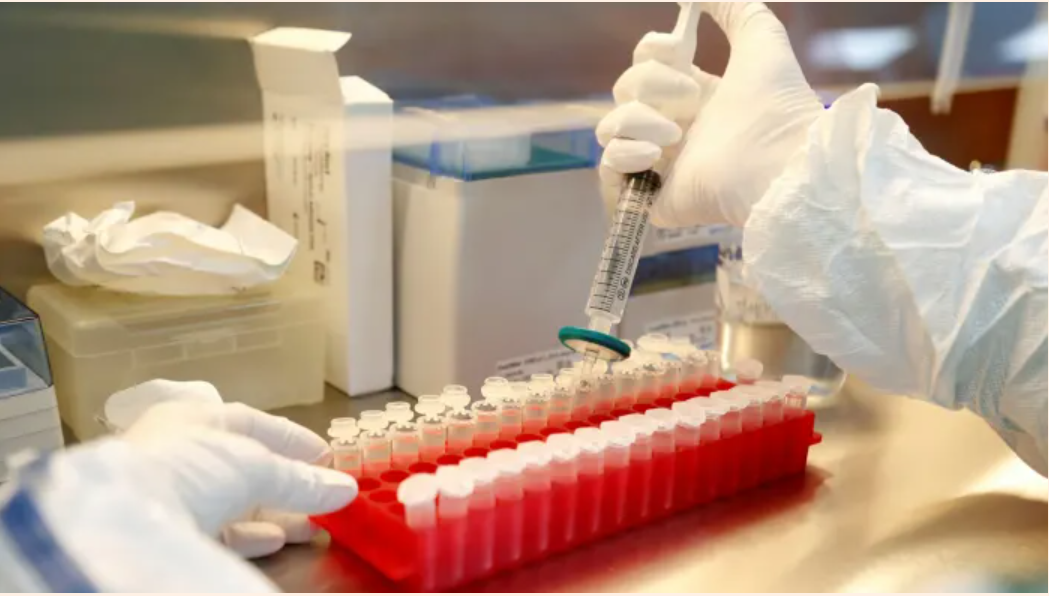
And that’s where ViroGates comes in. ViroGates’ suPAR test can identify those patients who, based on their inflammatory status, are expected to become even sicker. So when Sobi goes out to market Anakinra as a treatment for Covid-19 from October, Sobi’s sales people will simultaneously market and sell ViroGates’ suPAR test.
Virogates’ suPAR test is what is commonly called a biomarker, and while the percentage growth has been strong in recent years, the sales numbers have been small and modest. The successful launch of Anakinra and suPAR could turn that around. “There is no doubt that it will have even commercial significance for us if Sobi’s launch is successful,” says CEO Jacob Knudsen of ViroGates to Financial Weekly.
Sobi’s sales force, which sells only Anarkina and from October also suPAR from Virogates, is ten times larger than the sales force that normally sells and represents Virogates. The details of the application submitted are available on the EMA website, which can be viewed here.
ViroGates hopes that the marketing of suPAR with the new data, will make the many potential hospital customers aware of the opportunities they have to save money by generally using the suPAR test. In popular terms, the test can separate the sheep from the goats, so hospitals quickly send home those patients who do not require here-and-now treatment and who are costing hospitals dearly.
“For a small company like us, this is a great opportunity to gain an additional and invaluable entry point with current and future customers. When you make a living selling biomarkers, it is initially difficult to convince hospitals, but if it can be done in conjunction with a pharmaceutical product, it will be much easier,” Jacob Knudsen estimates.
ViroGates does not generally see itself as a Covid company, but international studies have shown that biomarkers can generally predict whether a patient will develop a serious disease or not, even on arrival in an emergency room. The study found that patients with high inflammation who received Anakinra became significantly less ill than those who received a placebo.
And it is these results, expected to be published soon, that have prompted Sobi to submit an application to the EMA.
ViroGate’s earnings budget for the remainder of 2021 is unlikely to be affected by Sobi’s application to the EMA, as the eventual approval will not take place until October.
Despite continuous losses, the company has maintained that its current capital position is sufficient until the company becomes cash positive.
After the Q1 results, CEO Jakob Knudsen said, “We are excited to start this year with strong revenue growth and the announcement of a ground-breaking study across 42 hospitals in Greece and Italy within the important field of combatting the COVID-19 disease. Also, we are happy to have welcomed four hospitals in Greece as new clinical routine customers – this holds much promise for the remainder of the year.”
The quarterly report did not disclose any information on the future collaboration with Sobi. The application to the EMA states that a conclusion on the evaluation can be expected in October and that the drug has been approved in the EU since 2002.
Morten W. Langer
Would you like to read more articles in English? Find them here: Our articles in English